IgG4 reagent for clinical analyzers
N-Assay LA IgG4 Nittobo
IgG4-related disease
IgG4-related diseases (IgG4-RD) have been recognized as a new concept of disease from Japan. Although the cause of IgG4-RD remains unclear, IgG4-RD have been associated with high levels of IgG4 in the blood and marked infiltration and fibrosis of IgG4-positive plasma cells in the affected organs, leading to simultaneous or heterogeneous enlargement, nodularity, and wall thickening in several human body organs, including the pancreas, bile ducts, lacrimal and salivary glands, thyroid, lungs, liver, digestive tract, kidneys, prostate, retroperitoneum, arteries, lymph nodes, skin, central nervous system organs, and mammary glands.
The lesions often involve multiple organs and manifest as a systemic disease, while single organ lesions can occur as well. Mikulicz’s disease and autoimmune pancreatitis are typical IgG4-RD. The clinical presentation is dependent on the location of the lesion, and the symptoms include increased organ size, obstruction due to wall thickening, spinal cord compression-associated symptoms, cellular infiltration, and fibrosis-related organ dysfunction, which can lead to serious complications.
Autoimmune mechanisms are considered to contribute to the pathophysiology of IgG4-RD, and first-line therapy comprises corticosteroids; however, IgG4-RD are intractable conditions that recur in many cases when the corticosteroid dose is reduced or discontinued.
*Based on Japan Intractable Diseases 300 (IgG4-RD) (Ministry of Health, Labour and Welfare)(https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000084783.html)
N-Assay LA IgG4 Nittobo
product description
- This reagent can be used in standard clinical analyzers.
- This reagent employs a monoclonal antibody for specifically measuring IgG4.
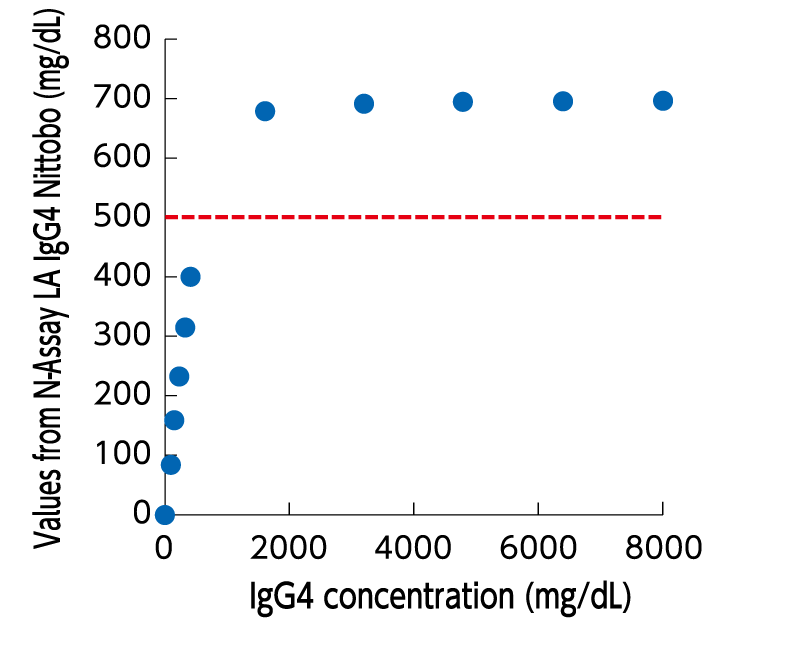
- This reagent employs a method that does not display false low values even at high IgG4 concentrations and can measure samples up to 500 mg/dl.
Superioradaptability and high IgG4 specificity
Enabling IgG4 measurement in laboratories withstandard clinical analyzers
The N-Assay LA IgG4 Nittobo is a reagent to measure serum IgG4 by clinical analyzers.
By using this reagent, any laboratory equipped with clinical analyzers can measure serum IgG4, facilitating quicker and easier implementation of the test.
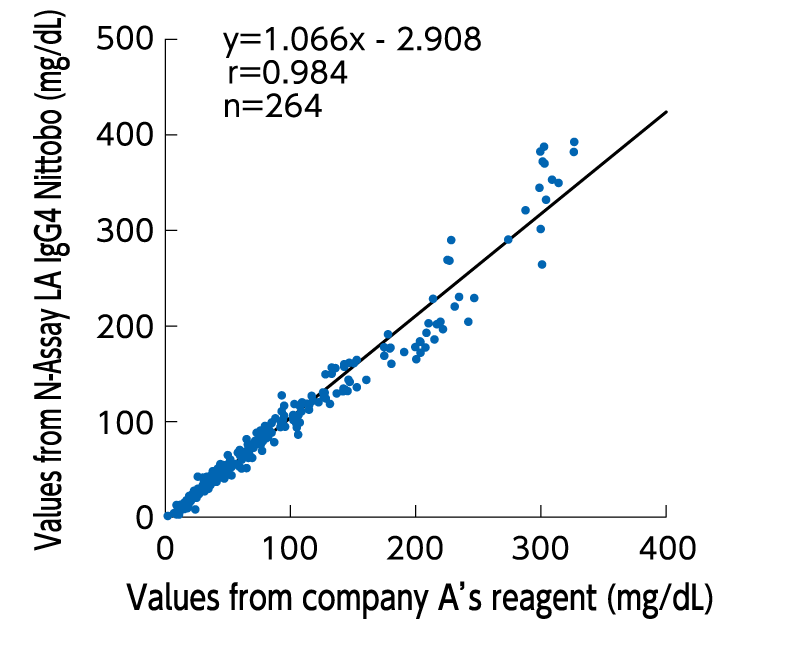
Correlation
Excellent correlation with existing reagents
No hook effect
This reagent was designed to employ the method that does not display false low values even in regions of high IgG4 concentration
Specify
Uses monoclonal antibodies with high specificity for IgG4
| IgG1 Myeloma | IgG2 Myeloma | Ig G3 Myeloma | |
|---|---|---|---|
| Theoretical value (mg/dL) | 3000 | 3000 | 3000 |
| Value from N-Assay LA IgG4 NIttobo (mg/dL) | 5 | 7 | 5 |
| Percentage of measurement value against theoretical value | 0.2% | 0.2% | 0.2% |
(Measured by JEOL BM9130-model clinical analyzer)
Reference range for N-Assay LA IgG4 Nittobo:11-121 mg/dL
*Usami Y, et al., Evaluation of a novel serum IgG4 assay and determination of reference interval for the Japanese population.Clin. Chim. Acta.501:136-141,2020